RIO DE JANEIRO, BRAZIL – Pfizer has announced that it has begun testing the safety in humans of a new pill against the coronavirus that could be used at the first symptoms of illness.
If proven successful in trials, the pill could be prescribed early in an infection to block viral multiplication before the patient’s condition worsens. The drug binds to an enzyme called protease to prevent the virus from replicating. Protease inhibitor drugs have had success in treating other types of viruses, including HIV and hepatitis C.

“Considering the mutated form of SARS-CoV-2 and the ongoing global impact of Covid-19, it will likely be critical to have access to therapeutic options now and after the pandemic,” Mikael Dolsten, chief scientific officer at Pfizer, said in a statement.
During an interview, Dolsten said that no unexpected problems have been observed so far and that the study could yield results in a matter of weeks.
The new protease inhibitor is the second such drug that Pfizer is testing in humans as a possible treatment for Covid-19. The company is testing another drug administered intravenously in hospitalized patients.
There is a lack of treatments that can be easily administered in the early stage of Covid-19. In the US, antibody treatments developed by Eli Lilly and Regeneron Pharmaceuticals are authorized for use in patients who have not yet been hospitalized but are at high risk of developing severe symptoms. These people must receive the infusion in the hospital or in a doctor’s office.
This restriction has created logistical challenges that have limited its use. Other therapies are aimed at sicker people: Gilead Sciences’ antiviral remdesivir needs to be given by infusion over several days and has only been approved for hospitalized patients.
Among the big pharma companies, Merck has developed one of the few pills against coronavirus that are already in human trials. Its experimental antiviral drug molnupiravir works with a different mechanism than the one used in Pfizer’s pill and is at an advanced stage of human testing.
Combined Phase Trials
If all continues to progress well, Pfizer could begin a broader trial combining phases 2 and 3 as early as the second quarter, Dolsten explained. This would potentially pave the way for the company to apply to the FDA (Food and Drug Administration) for emergency use authorization by the end of the year, depending on how the pandemic evolves.
The drug should be taken twice a day for about five days, he added. “It would really be a game-changer,” Dolsten said.
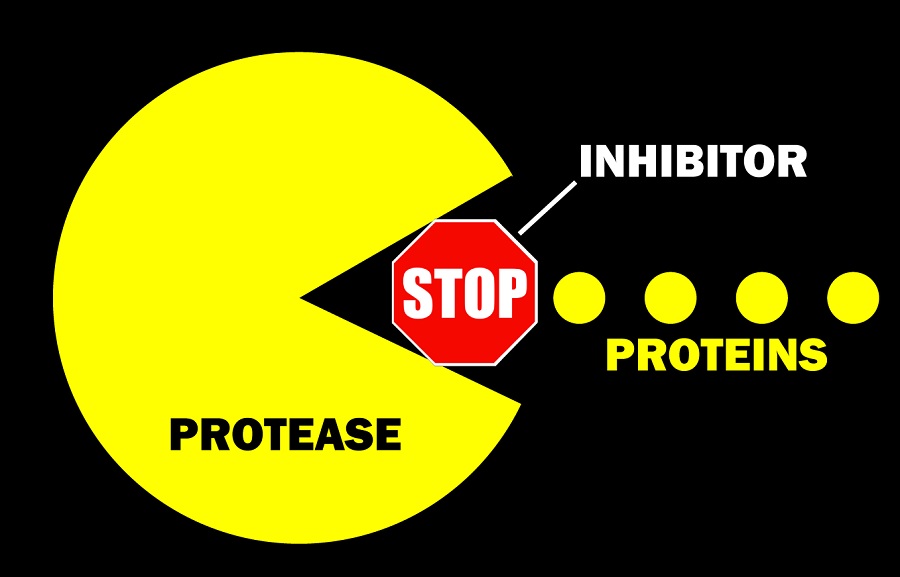
The initial efficacy test will focus on people with initial infection, but Pfizer also plans to study whether the drug works to protect healthy people who have been exposed to the coronavirus, such as family members or people living with someone who has become ill.
According to Dolsten, Pfizer’s oral protease inhibitor, codenamed PF-07321332, has a number of potential advantages. In laboratory tests, the drug worked against many coronaviruses, including SARS and MERS. In addition, the coronavirus protease does not mutate as much, which means that the therapy will likely work against multiple strains, he said.
In theory, the protease inhibitor could also be combined with other antiviral drugs, such as the one Merck is developing, Dolsten added.
Pfizer plans to share more data about the drug during the American Chemical Society meeting on April 6th.
Source: Infomoney

