RIO DE JANEIRO, BRAZIL – The Ministry of Health on Wednesday (22) reversed its recommendation to suspend Covid-19 vaccination of adolescents aged 12 to 17 without comorbidities.
The measure comes after the Brazilian Health Regulatory Agency (ANVISA) ruled out the death of a young girl in São Paulo as having been the result of an adverse effect.
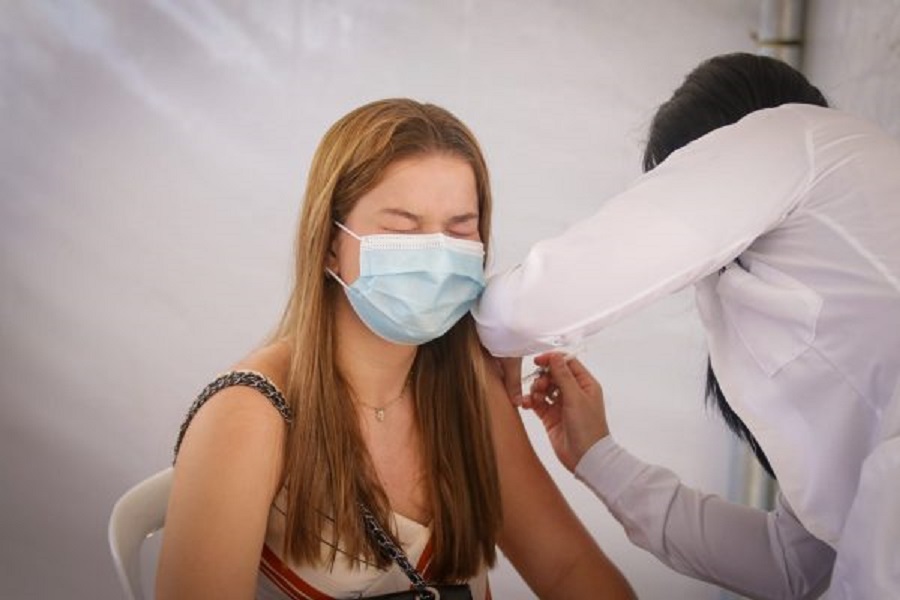
The executive secretary of the Ministry of Health Rodrigo Cruz stressed during a press conference that “the benefits of the vaccine are higher than the adverse effects.”
“Comparing everything that was administered, despite these alleged immunization defects, it is a very low percentage. The Ministry is no longer cautiously suspending immunization of adolescents with no comorbidities,” the executive secretary added.
The Ministry emphasized that adolescents may only be immunized with vaccines approved by ANVISA for this age group. Currently, only Pfizer is authorized.
According to ANVISA, the fatal case “was a clinical picture of thrombotic thrombocytopenic purpura (TTP), an autoimmune disease.”
The Ministry of Health issued a new technical note on the matter, published later on Wednesday. According to Ministry sources, the document has been in preparation since Tuesday, after the Federal Supreme Court (STF) said that states and municipalities have the autonomy to decide on the vaccination of this age group.
OTHER VACCINES
Another reason that led the Ministry to recommend the suspension last week was the alleged immunization of adolescents with vaccines other than Pfizer’s, the only one approved by ANVISA. “If there was an error in administration, surveillance is required,” Cruz said.

