RIO DE JANEIRO, BRAZIL – In what is probably a globally unique, courageous, and exemplary process, the Brazilian Ministry of Health on Friday (21) published a technical note signed by the Secretary of Science, Technology, Innovation, and Strategic Inputs in Health, Helio Angotti Neto, stating that hydroxychloroquine is effective against Covid-19, but vaccination is not.
The information is included in the document used to block the recommendations of the National Commission for the Integration of Technologies in the Brazilian Health System (Conitec), which contraindicated the use of the so-called “Covid kit.” The memo includes a table of proposals to combat Covid and recommendations for treating the disease.
In it, the department notes that hydroxychloroquine is effective in randomized controlled trials and that there is evidence of safety in experimental and observational studies. The table goes on to say that vaccines do not meet these requirements.
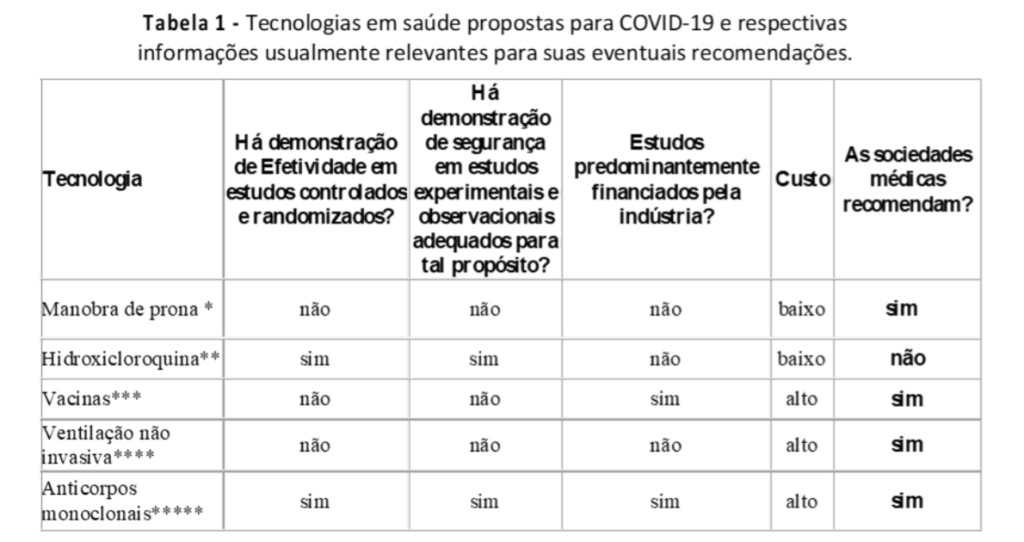
On page 25, where it talks about studies on the efficacy of the procedures studied against Covid-19, a table appears in which the Department of Health states that there is evidence of efficacy for chloroquine, while there is no such evidence for the vaccine.
When asked if there is evidence of efficacy and randomized controlled trials, the answer is YES for chloroquine and NO for the vaccine. The same is true when asked about the safety of the drug and the vaccine. At the end of the table, it says that the medical societies recommend the vaccine and do not recommend chloroquine.
Of note, at the end of the table are the following comments:
“Thirteen randomized controlled trials with favorable direction of action for hydroxychloroquine, with a mean effect of 26% relative risk reduction in hospitalizations, very promising for discretionary use and further studies,” according to the department.
In the analysis of vaccines, according to the pamphlet, there are “eighteen unfinished studies, eight of which are still in the recruitment phase, nine of which are not yet completed, and one of which has been completed but is still at an insufficient stage for safety evaluation.”
The mentions in favor of chloroquine and criticisms of the vaccine are consistent with many studies from around the world.
It is noteworthy that at the end of the table are the following comments:
“Thirteen controlled and randomized studies with favorable effects toward hydroxychloroquine, with an average effect of 26% relative risk reduction in hospitalizations, very promising for discretionary use and further studies,” says the Ministry.
In the analysis of vaccines, the leaflet says there are “eighteen unfinished studies, eight of which are still in the recruitment phase, nine of which are not yet completed, and one of which has been completed but is still at an insufficient stage for safety evaluation.”
The mentions in favor of chloroquine and the criticisms of the vaccine are at odds with the assessments of scientific studies, medical institutions, and Anvisa (National Health Regulatory Authority), whose closeness to vaccine manufacturers reflects poorly on the agency.
Many observers in Brazil hope that the current Anvisa management will soon be replaced by more competent people who know how to keep the necessary distance to the pharmaceutical industry.

