RIO DE JANEIRO, BRAZIL – The decision of the European Medicines Agency (EMA) not to include Covishield, AstraZeneca’s version of the vaccine manufactured in India, in a list of immunizers approved for its digital certificate for travelers, has caused doubts regarding the entry into the European Union of citizens from countries outside the block.
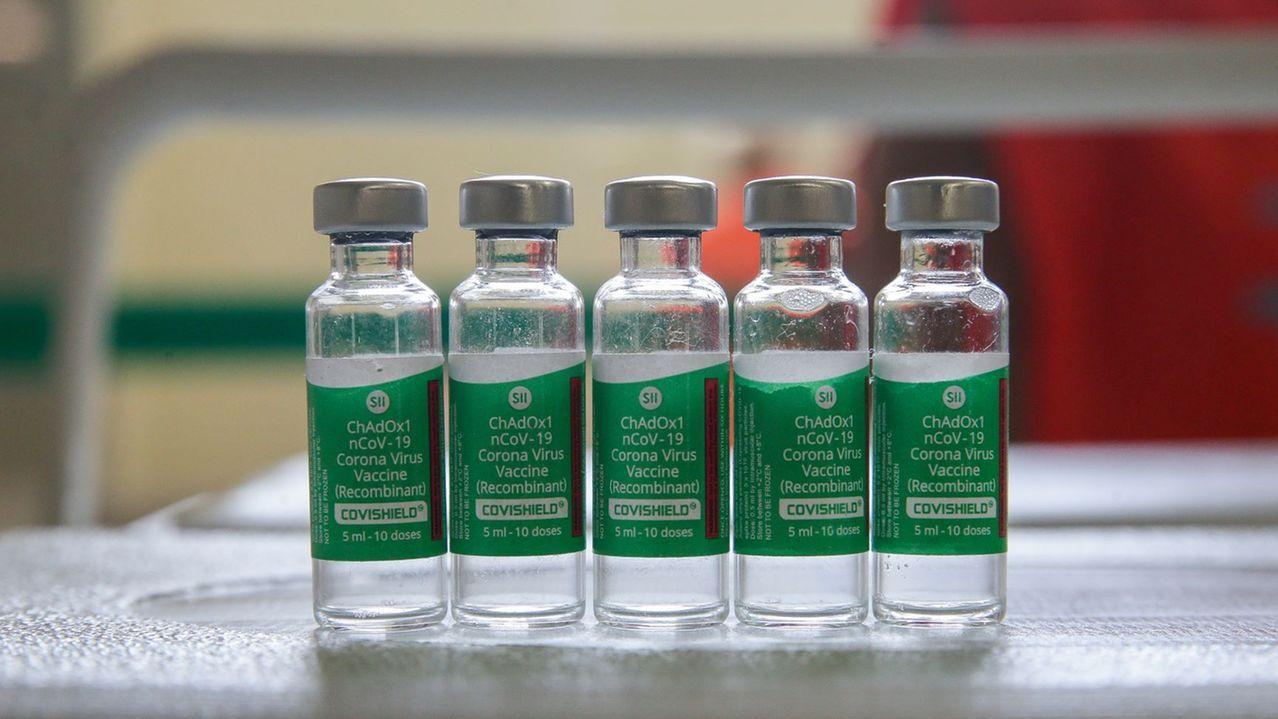
Brazil’s Fiocruz received 4 million ready doses of Covishield among the 66 million doses of the AstraZeneca immunizer – which would expose citizens of Brazil to difficulties when traveling to Europe.
On Tuesday, June 29, when the agency announced the decision due to differences between Covishield’s formula and AstraZeneca’s original, the African Union criticized the measure, which it called “unfair.”
“The availability of the European certificate, with its potential to significantly facilitate free and safe movement throughout EU member states and some associated countries, is a significant advance,” the African entity said in a statement.
“However, the current applicability guidelines put at risk the equitable treatment of people who have received certain versions of vaccines distributed by the EU-backed Covax Consortium, such as most African Union member states.”
The statement points out that Covax distributes Covishield, produced by the Serum Institute. Still, with the exclusion of the EMA, “people who received this immunizer, despite being able to prove vaccination, would continue to be subject to public health restrictions, including movement limitations and testing requirements, with considerable administrative and financial implications.”
AstraZeneca’s vaccine was developed in partnership with Oxford University and is produced under license by several laboratories, including India’s Serum, South Korea’s Bioscience, and Fiocruz in Rio de Janeiro.
Vaccination certificates in Brazil specify the brand name but not the origin of the immunizer administered – which could expose everyone who received AstraZeneca vaccines to the effects of the European veto. So far, Fiocruz has not commented on the case.
Source: Valor

