RIO DE JANEIRO, BRAZIL – Minister of Health Eduardo Pazuello on Thursday, January 7th, announced the signing of a contract with the Butantan Institute for the supply of 100 million doses of the Covid-19 vaccine – 46 million by April and another 54 million doses by the end of the year.
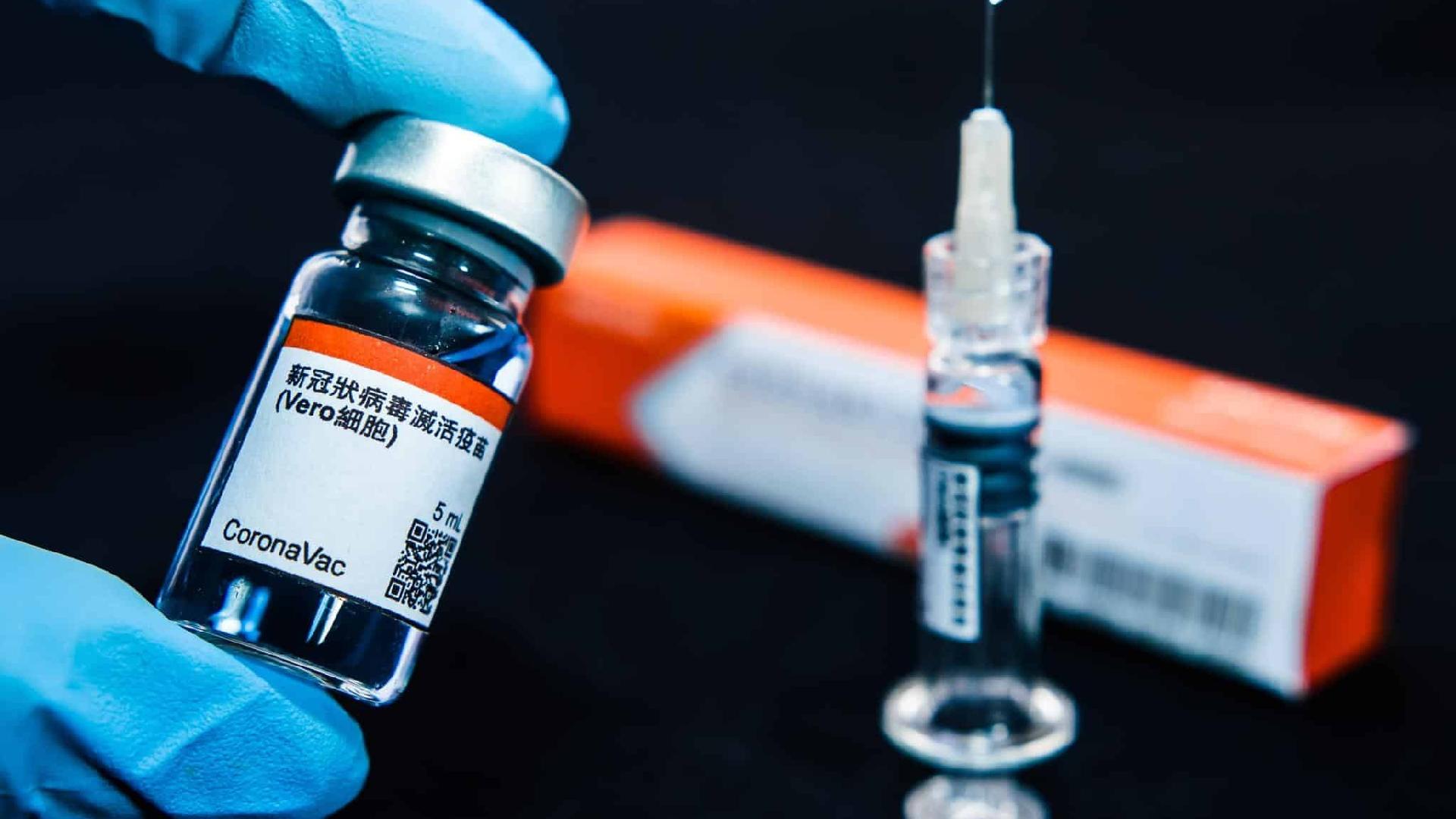
According to the Minister, all Butantan production will be incorporated into the National Immunization Plan, for nationwide distribution. According to Pazuello, the price for the dose is a little over US$10.
Pazuello gave the statement during a press conference convened by the federal government to explain the Provisional Measure announced the day before by the Minister himself which provides “exceptional measures” for the purchase of vaccines, supplies, goods and logistics services for vaccination.
In a statement, the Butantan Institute reported that it has received the draft contract with the Ministry and forwarded it for review by its legal department “aiming for its speedy completion”.
Ministry of Health Executive Secretary Elcio Franco said that the contract between the Ministry and the Butantan was intended for the purchase of 46 million doses of the vaccine and the purchase option for another 54 million doses. According to Franco, the purchase of the full 100 million doses will not yet be formalized because the Ministry of Health lacks sufficient budget for the purchase at this time.
Earlier on Thursday, the São Paulo government announced that the CoronaVac vaccine, developed by the Butantan in partnership with Sinovac Chinese Pharmaceutical company, has recorded 78% efficacy in clinical trials conducted in Brazil.
São Paulo governor João Dória stated that the Butantan will submit a request to the National Health Regulatory Agency (ANVISA ) for the Coronavac’s emergency use on Friday, January 8th. According to ANVISA, the term for the emergency use request review is ten days. The definitive registration request assessment is done in up to 60 days.
Pazuello also said the Ministry is negotiating vaccine procurement with international laboratories. According to him, negotiations with Jansen provide for the supply of three million doses in the second quarter. “Unfortunately,” he said, “we are only offered three million doses.”
“The fact that it is manufactured in Brazil is what suits us. If not manufactured in Brazil, the amounts will always be minimal compared to Brazil’s needs,” he said.
The Minister said that Pfizer offered 500,000 doses in January, 500,000 in February and two million in March, April, May and June. In the case of the Moderna vaccine, Pazuello said that the delivery of 30 million doses – at US$37 a dose – is expected from October.
“Think if this solves the problem of Brazil. Every vaccine offered by Pfizer in the first semester will vaccinate half of the population of Greater Rio de Janeiro. Eight million doses, four million people vaccinated,” he said.
Pazuello also mentioned negotiations with União Química for the manufacture in Brazil of the Sputnik-V vaccine, from the Russian Gamaleya laboratory.
In total, Pazuello said that Brazil has secured 354 million doses of vaccines for 2021, distributed as follows:
– Two million doses of AstraZeneca’s vaccine imported by the Oswaldo Cruz Foundation (Fiocruz);
– 100.4 million doses of Fiocruz/AstraZeneca until July (national production with imported active pharmaceutical ingredient (IFA);
– 110 million doses from Fiocruz/AstraZeneca (full national production) between August and December;
– 42,5 million (probably from AstraZeneca) to be acquired through the international Covax/Facility;
– 100 million doses from the Butantan Institute.
Pazuello said that the vaccination against Covid-19 will start between January 20th and early March. According to him, this will depend on the records and vaccine production.
“I will give you three periods. First period, until January 20th, at best. We are counting on the Butantan’s vaccines – in case ANVISA authorizes us to use them – we are counting on the vaccines imported from AstraZeneca – in case all this is correctly processed and ANVISA authorizes us to use them”, he declared.
“In the average scenario, it would be from January 20th to February 10th. We are counting on the vaccines produced in Brazil, both at Butantan and Fiocruz. And in the most lengthy scenario, from February 10th until the beginning of March, which would be the case should the registration and production experience any setbacks,” said Pazuello.

