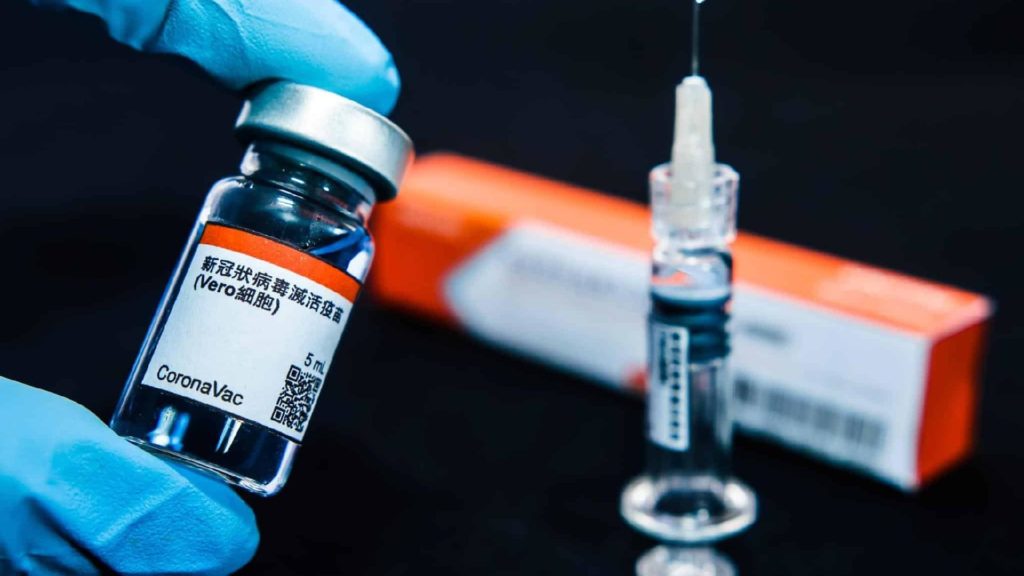
RIO DE JANEIRO, BRAZIL – The cause of death of the CoronaVac vaccine volunteer was suicide, according to the police report obtained on Tuesday, November 10th, by TV Globo.
The previous day, the National Health Regulatory Agency (ANVISA) temporarily suspended the vaccine trials, developed by the Chinese pharmaceutical company SinoVac. In Brazil, production of the vaccine will be under the responsibility of the Butantan Institute, which is linked to the government of São Paulo and is also coordinating the CoronaVac trials in the country.
When announcing the interruption of trials, the ANVISA mentioned a “serious adverse event”, but failed to provide details about the specific reason leading to the suspension.
Shortly after the announcement of the volunteer’s cause of death, ANVISA’s director-president Antonio Barra Torres stated at a press conference that “there was no official information [that the volunteer had committed suicide] among the reports we received yesterday [Monday]”.
According to the police report registered at 4:02 PM on October 29th in a police station in the West Zone of São Paulo, State Police officers were called by radio to attend to a “dead body finding occurrence”.
Upon reaching the apartment, the police officers were met by the building’s janitor, who led them to a 32-year-old man on the bathroom floor – near his arm there was a syringe and several medicine vials. The young man’s body was forwarded to the Forensic Institute (IML).
The autopsy report had not been released by the last update of this article. The final result is dependent on the toxicological examination, which takes longer to be completed.
With the interruption of the CoronaVac trial, no new volunteer may be vaccinated in Brazil.
The São Paulo government has agreed to purchase 46 million doses of the CoronaVac, which was at the heart of a dispute involving Jair Bolsonaro, the Ministry of Health and São Paulo Governor João Doria, the President’s political opponent.
According to the World Health Organization, there are currently ten vaccines in their third and final stage of human trials – among them the CoronaVac.
Before being released to the population, a vaccine must undergo three stages of clinical trials to prove its safety and efficacy. At each stage, more volunteers are recruited, and the trial results are analyzed by researchers to ensure that the immunizer can be licensed.
Serious adverse event
On Monday night, ANVISA suspended the CoronaVac vaccine trials after being notified of a “serious adverse event” on October 29th, involving a volunteer.
More than ten days later, the agency determined that no new volunteer may be vaccinated until the data are assessed and the ANVISA may “evaluate the risk/benefit of continuing the trial”.
According to a list disclosed by the agency, serious adverse events are considered “death, potentially fatal adverse event, disability or persistent disability, hospitalization of the patient, congenital anomaly or birth defect, any suspicion of transmission of an infectious agent through a medical device and a clinically significant event”.
On Tuesday, ANVISA said that the decision to suspend the CoronaVac trials was “technical” and based on the absence of information.
The Butantan says there is no link between the vaccine and the death
After the announcement of the suspension of trials, the Butantan Institute director Dimas Covas, said:
“The data are transparent. Why do we know and are certain that this event is not related to the vaccine? As I said, from the clinical perspective of the case – and we can not provide details, unfortunately – it is impossible, it is impossible to relate this event to the vaccine, impossible. I think this definition ends this discussion somewhat.”
The statement was made by the director during a press conference at the Butantan Institute headquarters after the São Paulo government met virtually with ANVISA officials to discuss the suspension of trials.
On Monday night, Dimas Covas had already stated that the volunteer had died, but said the death was unrelated to the vaccine.
During Tuesday’s press conference, São Paulo government officials protested against the suspension and argued that there is no link between the adverse event and the immunizer.
Dimas Covas said that the suspension, in addition to unnecessary, causes “pain and suffering in volunteers”: “There would be no need for this type of measure, which could be resolved administratively, as was done this morning.”
“If interrupting a clinical trial that is going very well causes suffering, causes pain, causes insecurity in those who have already been submitted to the trial, it leads to hardship for those who want to be submitted to the trial and who are waiting in line to be administered a vaccine or a placebo. They are the volunteers, the people who dedicated themselves to this trial to enable hope for a vaccine.”

ANVISA was notified in early November
Also according to Dimas Covas, ANVISA was notified on November 6th of the adverse event involving the CoronaVac trial volunteer.
“We are dealing with a serious adverse event that has no relation to the vaccine. I repeat: a serious adverse event that has no relation to the vaccine. This data has been made available to ANVISA since November 6th, when the serious adverse effect was notified”, said Covas.
The Butantan director criticized the agency’s conduct and the way the institute was notified of the interruption of trials.
“On November 6th, ANVISA received a document stating: ‘A participant of the clinical trial experienced a serious adverse event unrelated to the vaccine’. Period. What is expected before such a report? ‘Look, ok, let’s assess it, let’s meet, let’s see what were the causes of this adverse event if you are saying that it is unrelated to the vaccine, let’s find out’, he explained.
“That’s what we expect. Is that what happened? No. I mean, this referral was made on the 6th. Yesterday, November 9th, at 8:40 PM, they sent the Butantan an e-mail saying that there would be a meeting today to discuss the serious adverse event. But, in parallel, they announced the trial was suspended. At 8:40 PM, 20 minutes later this news was on national television. Twenty minutes after we were notified by e-mail, the news was on national television”.
Dimas Covas also said that the government has forwarded all clarifications to ANVISA and is waiting for clearance to resume trials as soon as possible: “It is now prepared to make the decision to restart trials as soon as possible”.
The director expects that, after the clarifications, ANVISA will allow restarting trials this week, possibly by Wednesday, November 11th.
Sinovac Press Release
On Tuesday, Sinovac, the Chinese pharmaceutical company behind the development of the CoronaVac, said in a statement that it “is confident in the safety of the vaccine” against Covid-19.
The company stated that Phase 3 clinical trials in Brazil “are conducted strictly according to the requirements of GCP” (Good Clinical Practices).
Oxford vaccine
On October 21st, a Brazilian volunteer participating in the Oxford vaccine trials against Covid-19 died from Covid-19 complications. The volunteer had been given a placebo (inactive substance), not a vaccine dose. The trials were suspended at the time by AstraZeneca itself, which is developing the vaccine in partnership with Oxford University.
Source: G1

