RIO DE JANEIRO, BRAZIL – On Tuesday, August 11th, Russian President Vladimir Putin announced that Russia is the first country in the world to register a vaccine against the novel coronavirus causing Covid-19. Despite the announcement, little is known about the efficacy of this vaccine, and it has been challenged by international scientists.
Russia has also announced that Brazil should be involved in the next phase of vaccine trials, scheduled to begin on Wednesday.
“This morning a vaccine against the novel coronavirus was registered for the first time in the world,” said the head of the Kremlin in a meeting with his Cabinet.

According to the August 10th update from the World Health Organization (W.H.O.) on vaccines under development against Covid-19 in the world, the Russian vaccine was still in phase 1 of testing. The development of a vaccine always comprises 3 stages.
On Tuesday, August 11th, the W.H.O. commented on the Russian vaccine announcement. It stated that Russia “does not need its approval” to register the vaccine and that it [W.H.O.] will need access to research data to assess the vaccine’s efficacy and safety in order to approve it.”
In all, 165 vaccines against Covid-19 are being researched worldwide, according to the health organization’s data. Six of these candidates are in their final phase of human trials (phase 3).
The Russian vaccine will be called Sputnik V, in reference to the Cold War space race between the Soviet Union and the United States. Sputnik I was the first satellite to orbit the Earth, launched by the Soviets in 1957.
Partnership with Brazil
On Tuesday, August 11th, the government of Paraná announced that it would sign an agreement to manufacture the Russian vaccine. However, production is not scheduled to begin, as it requires clearance from Brazil’s Health Regulatory Agency ANVISA.
On July 24th, the state government had already announced that it was studying a partnership with Russia to manufacture the vaccine.
Asked about the vaccine production, Fiocruz replied that “the agreement with the Russian institute for vaccine production will be made with the Institute of Technology of Paraná (TECPAR), as stated by the institute’s director this morning.”
Fiocruz has a partnership agreement with pharmaceutical company AstraZeneca, in the United Kingdom, for technology transfer to Brazil for production of the vaccine produced by Oxford University.
According to the new Russian vaccine’s website, Brazil will be involved in phase 3 of the clinical trials, which are scheduled to begin on Wednesday, August 12th. There will be two thousand subjects involved; in addition to Brazilians, there should be volunteers from Russia, the Arab Emirates, Saudi Arabia, and Mexico.
The Ministry of Health was asked about possible Brazilian involvement in the tests. Through the National Health Regulatory Agency (ANVISA), the government stated that there is no request from any organization or partner regarding this vaccine in the country.
According to ANVISA, conducting a clinical trial in Brazil requires the approval of the National Commission of Ethics in Research (CONEP), an entity linked to the Ministry of Health.
Mass production of the vaccine in Russian territory is expected to begin in September.
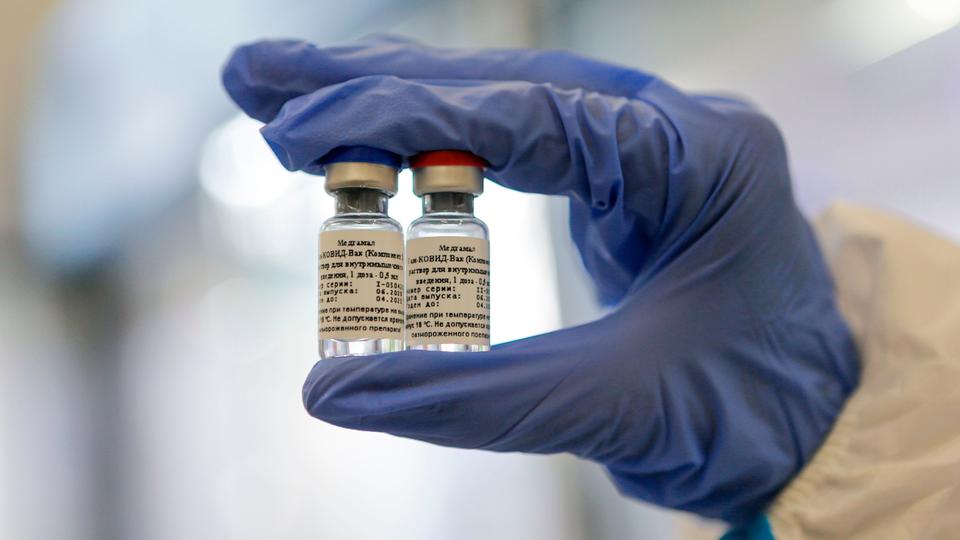
What is known about the Russian vaccine?
The vaccine was developed by the National Research Center for Epidemiology and Microbiology of the Ministry of Defense.
According to the vaccine’s website, it was tested on animals before being administered in humans – including two types of primates. However, Russia has not published any scientific studies on the tests it has carried out.
Human trials began on June 18th, when the first group of 18 volunteers was immunized in its lyophilized form (lyophilization is a kind of “vaccine dehydration” that stabilizes the molecule). Five days later, on June 23rd, a further 20 people were administered the dose, also of this type.
According to the details available online on the vaccine tests (in the lyophilized and liquid version), the first 18 volunteers of the lyophilized form were split into two groups of nine. Each group received a single dose of the vaccine; the difference among them was the adenovirus which served as the “carrier” for the novel coronavirus protein. This step corresponded to phase 1 of the trials.
Phase 2 consisted of 20 volunteers, who began trials on June 23rd. These people were administered a booster in addition to the first dose, which was scheduled for 21 days after the first dose. Unlike the first 18, all 20 phase-2 volunteers were administered both vaccines (each with a type of adenovirus).
In July, Russia announced the vaccine-induced antibody production in the first phase of testing. In the same month, Sechenov University in Moscow reported that another version of the same vaccine, in liquid form, was being tested on 38 other volunteers at a military hospital in the Russian capital. The volunteers were split up in the same way as the freeze-dried vaccine.
Phase 1 and 2 testings were completed on August 1st, according to the official vaccine website.
“No subjects in the current clinical trials have been infected with Covid-19 after having the vaccine administered,” the website says.
“The high efficacy of the vaccine has been confirmed by high-precision antibody tests in the volunteers’ blood serum (including a test for antibodies that neutralize the coronavirus), as well as the volunteers’ immune cells’ ability to be triggered in response to the coronavirus S protein, which points to antibody formation and cellular immune response,” the text states.
Felix Ershov, a member of the Russian Academy of Sciences, said the vaccine is safe and effective.
“The safety of the vaccine is guaranteed because it uses a cold virus that is harmless to humans and does not contain the coronavirus itself, and only a part of its genetic code is present, thus ruling out the possibility of infection,” he said.
“But the production of the antibodies needed to protect the body is guaranteed, which is not only evidenced in the test results, but also in the use of other vaccines of this type,” Ershov added.
“This vaccine is not as integral as earlier vaccines (live or inactivated virus). It was designed with advanced biotechnology,” he added.
Sergey Tsarenko, deputy director of Anesthesiology and Resuscitation at Moscow Hospital No. 52, compared the vaccine’s operating mechanism to launching a spacecraft.
“An orbital station, a filament of the coronavirus, is linked to the adenovirus, harmless to humans, [which functions] as a launching vehicle, and can also be launched inside the human body,” he said.
“After that, immunity is developed for both the ‘launching vehicle’ and the ‘orbital station’. Then, three weeks later, the same ‘orbital station’ is launched into another ‘launching vehicle’, that is, a different adenovirus,” explained Tsarenko.
“Several other vector vaccines are being developed around the world, but so far no one has considered using two ‘launching vehicles’ to achieve this goal. In addition, the first test subjects were employees of the Gamaleya Institute,” he added.
“The vaccine was then tested on other volunteers. There wasn’t a single complication and all the participants displayed a powerful immune response,” said Tsarenko.
Russian President Vladimir Putin said the Russian vaccine is “effective”, has passed all the required tests, and provides a “stable immunity” against Covid-19.
International agencies also report that the Russian president said one of his daughters has already been administered the vaccine. His daughters are Maria, 35, and Ekaterina, 34, but there is no information as to which of them has been vaccinated.
The W.H.O. stressed the importance of 3 test phases
On Monday, August 10th, the W.H.O. alerted that although there are several vaccines in their final testing phase, their efficacy still needs to be demonstrated, and that there will probably not be an “immediate solution”.
“Several vaccines are now in phase three clinical trials, and we all hope to have several effective ones that can help prevent infection in people. However, there is no immediate solution at this time and there may never be,” said Director-General Tedros Adhanom Ghebreyesus.
Vaccination in Russia
According to the Russian Ministry of Health, after registration and production, vaccination should begin in October free of charge. Initially, according to the health authorities, special groups within the population will be vaccinated: doctors, teachers, and those who are in constant contact with large groups of people.
On Monday, August 10th, in an interview with Itar-Tass, the Minister of Industry and Trade, Denis Manturov, said that next month three Russian companies will start commercial production.
Source: G1

