RIO DE JANEIRO, BRAZIL – Over one million people have applied to volunteer for the Coronavac trials, a Chinese vaccine that has been tested in Brazil since last week and is the result of a partnership between the Butantan Institute and Sinovac Biotech. The announcement was made by the state Secretary of Health, Jean Gorinchteyn.
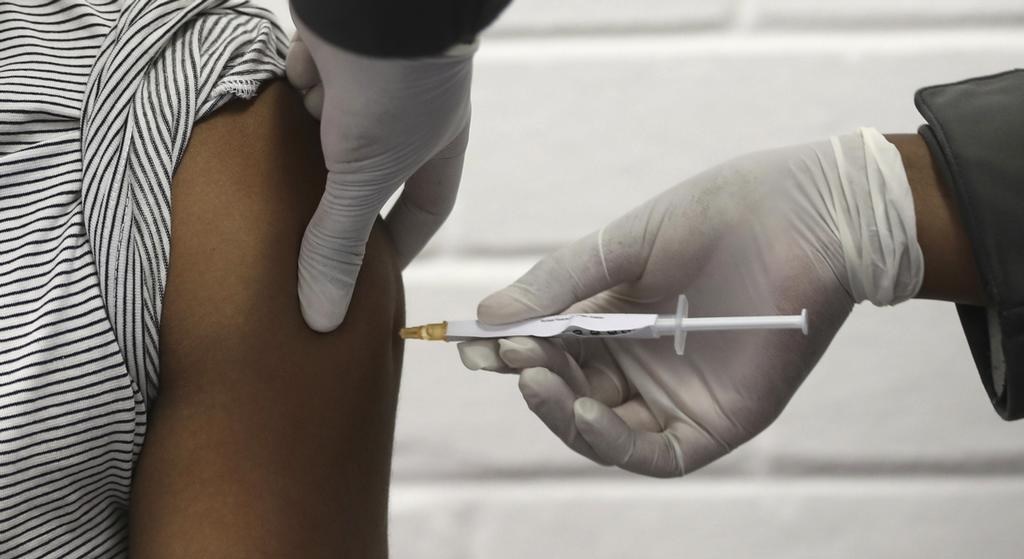
The vaccine began to be tested last Tuesday on volunteers at the Clínicas Hospital in São Paulo. And from this Thursday and Friday, July 30th and 31st, the vaccine will be tested in four other centers: Emílio Ribas Institute in São Paulo; Clínicas Hospital of Ribeirão Preto; São Caetano do Sul Municipal University; and Federal University of Minas Gerais Drug Research and Development Center in Belo Horizonte.
In all, 9000 volunteers, healthcare professionals only, will be administered the vaccine at 11 research centers. In the capital city of São Paulo, the Emílio Ribas Infectology Institute and the Albert Einstein Israelite Hospital will also be involved in the study. Furthermore, the São Caetano do Sul Municipal University, the Clínicas Hospital of UNICAMP (University of Campinas), the Rio Preto Medical School and the Ribeirão Preto Medical School Health Center were selected in the State of São Paulo. The study will also be conducted in centers in Belo Horizonte (Federal University of Minas Gerais – UFMG), Rio de Janeiro (Fiocruz), Brasília (University of Brasília – UNB), Curitiba (Federal University of Paraná – UFPr), and Porto Alegre (Pontifical Catholic University of Rio Grande do Sul – PUC-RS).
To be eligible to take part in the trial, applicants must not have contracted the novel coronavirus, or have taken part in other studies. Women must not be pregnant or planning a pregnancy within the next three months. A further restriction is not to have conditions requiring medications that could alter the immune response. Among those recruited, half are administered two doses of the immunizer in a 14-day interval and the other half are administered two doses of a placebo.
The government estimates that the study should be completed by September. Should the trials be successful, the vaccine could enter production in early 2021.
The first dose was administered to 890 employees of the Clínicas Hospital on Tuesday, July 21st. They will be provided with a second dose within 14 days and will be monitored by doctors.
According to the state government, the Butantan Institute is adapting its manufacturing facility for the production of the vaccine, with a production capacity of up to 100 million doses. The agreement with the Chinese laboratory provides that should the vaccine prove effective, Brazil will initially be supplied with 60 million doses manufactured in China for distribution.
Source: Estadão Conteúdo

