RIO DE JANEIRO, BRAZIL – The Ministry of Health may authorize, until Tuesday, March 24th, the prescription of chloroquine and hydroxychloroquine for severe Covid-19 cases, a disease caused by the novel coronavirus.
The statement was made earlier by the portfolio’s executive secretary, João Gabbardo dos Reis. Until then, the ministry will release a note with guidelines on the use of the drugs.
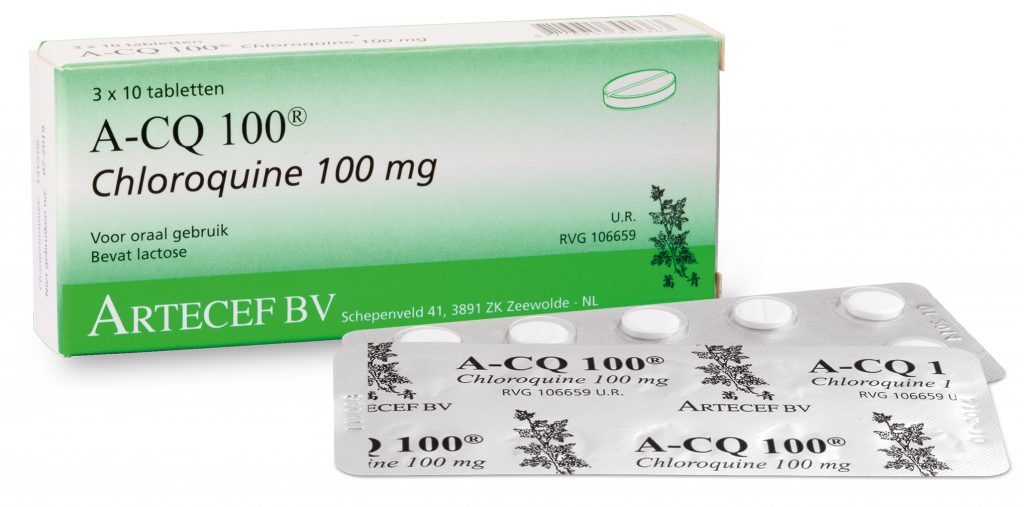
However, the secretary stated that the potential release of the drugs will be experimental and will only apply to patients hospitalized in critical condition. He reiterated that the two components have strong side effects and cannot be stored to be used in potential flu cases.
“Today, [the drugs] are used in clinical research, with authorization from the hospitals’ ethics committees, in association with other drugs. If the Ministry of Health releases the prescription, it can be used for critically ill patients in hospitals. It’s not to be used by people who have the flu and who think that if they take this drug, they won’t have complications,” Gabbardo says.
In recent days, a study conducted in France was released in which chloroquine – used to treat malaria – and hydroxychloroquine – prescribed for cases of rheumatoid arthritis and lupus – decreased the viral count. On Friday, Match 20th, US President Donald Trump reported that the US government is studying the use of the drugs to treat the new coronavirus.
Regarding President Jair Bolsonaro’s authorization for the Army to increase drug production, the executive secretary said the measure was preventive in the event of a potential increase in future demand. In Brazil, the product is manufactured in private laboratories, the Armed Forces and the Oswaldo Cruz Foundation (Fiocruz).
“These drugs are already manufactured in Brazil and exist in pharmacies. Due to the potential use for severe cases of coronavirus, we are considering the need to increase production. This is what the President authorized: that the Army may increase the production of drugs,” he explained. He recalled that the National Health Surveillance Agency (ANVISA) has restricted the sale of drugs to those people with medical attestation that they have the three diseases now treated by the drugs: malaria, lupus, and rheumatoid arthritis.
Vaccination
Regarding the influenza vaccination campaign, which begins on Monday, March 23rd, for seniors and health professionals, Health Surveillance Secretary Wanderson Kleber de Oliveira reported initiatives by states and municipalities to prevent the crowding of seniors in health facilities. He mentioned partnerships with schools for vaccination in courtyards, better-ventilated environments, and agreements with drugstores, companies, and organizations in the productive sector to prevent the concentration of people in a single place.
Oliveira mentioned initiatives for health professionals to take the doses to hospital facilities and clinics for colleagues to be vaccinated. This, the secretary said, prevents the mass displacement of health professionals to vaccination posts. He further mentioned an initiative by the government of Pará, which will perform a drive-thru vaccination system in which the driver does not get out of the car.
The secretary reiterated the recommendation for local governments to defer vaccination of children. The campaign begins this Monday for the elderly and health professionals. In the subsequent stage, public safety professionals, the chronically ill and people with restricted freedom will be vaccinated. In its last stage, vaccination will only be available to the remaining people. He mentioned the streamlining of registration, collecting only the vaccine record, not the patient’s name, to reduce the length of stay in the facility.
Source: Exame

