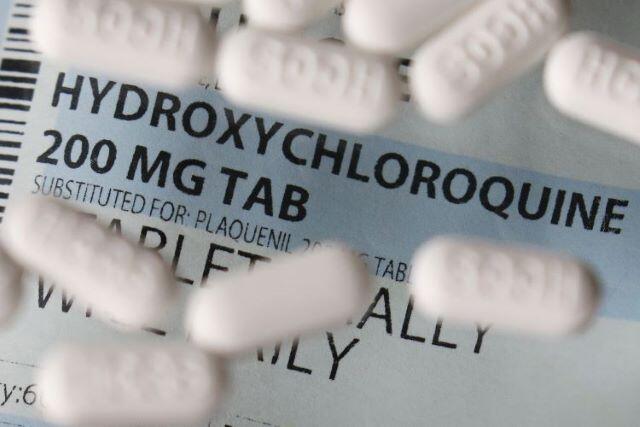
RIO DE JANEIRO, BRAZIL – On Monday, May 25th, the World Health Organization (WHO) suspended the use of hydroxychloroquine in research it had been coordinating with scientists from 100 countries.
The temporary suspension was decided until the drug’s safety is reassessed, since recent studies have shown that it is not effective against Covid-19 and may even increase the mortality rate.

The WHO says that the remaining trials are continuing within the international initiative called “Solidarity”. In addition to the drug now vetoed, researchers are also evaluating the results of three types of antivirals and one drug used to treat multiple sclerosis in patients (read more below).
According to WHO chief scientist Soumya Swaminathan, chloroquine is not used in the Solidarity initiative trials. Both chloroquine and hydroxychloroquine use the same active ingredient, but chloroquine is considered potentially more toxic. Hydroxychloroquine, consisting of an “attenuated” version of the substance, is considered safer and is used in long-term therapies.
Tedros Ghebreyesus, the WHO Director-General, said the suspension was decided after the release of the study published on Friday, May 22nd, in the scientific journal “The Lancet”. The study, conducted with 96,000 people, pointed out that the substances were not effective against Covid-19 and detected risks of cardiac arrhythmia in patients who used them.
WHO had already announced that it was against the widespread use of chloroquine to treat Covid-19. When Brazil began to recommend that patients with mild conditions could use the drug, the organization’s Directors stressed that the drug should only be used within “clinical trials”, which are tests within medical research frameworks.
“The authors reported that among Covid-19 patients using the drug, alone or using a macrolide [class of antibiotics of which azithromycin is included], they estimated a higher mortality rate,” Tedros said.
WHO said Solidarity’s executive board will analyze globally available data on the drugs, which are used to treat malaria and autoimmune diseases.
“I want to reiterate that these drugs (chloroquine and hydroxychloroquine) are accepted as generally safe for use in patients with autoimmune diseases or malaria,” Tedros said.
Tedros added that the other Solidarity trials will continue (see details below) – the suspension refers only to chloroquine and hydroxychloroquine research.
Solidarity trials
The Solidarity trials were announced by Tedros on March 18th. Several hospitals around the world are part of the initiative. According to the organization, on Monday, May 25th, there were 35 countries recruiting patients for trials in over 400 hospitals worldwide.
According to WHO, the initiative may reduce the time needed for clinical trials by 80 percent, which usually takes years to be designed and conducted.
Any adult with Covid-19 who is admitted to a participating hospital may be part of the research. Patients are randomly assigned by a computer to five different treatment options:
A group of patients is given only the standard form of treatment from where they are based.The second group is given this form of treatment + the remivir antiviral, which has already been tested for Ebola and had promising results against both SARS and MERS, also caused by coronaviruses (such as Sars-CoV-2, the novel coronavirus).
The third group is given the standard treatment + chloroquine or hydroxychloroquine (this was the suspended “branch” of the research).
The fourth group is given standard treatment + lopinavir and ritonavir antivirals, used to treat HIV. There is still no evidence that they are effective in treating or preventing Covid-19, according to the WHO.
The fifth group receives standard treatment + interferon beta-1a, used to treat multiple sclerosis.
Before the randomized assignment of treatment, the patient is assessed by a medical team to rule out medicines that could not be administered to him or her.
In Brazil, the Solidarity trials are coordinated by Fiocruz in Rio de Janeiro.
Chloroquine and hydroxychloroquine in Brazil
Despite the lack of scientific evidence to prove the efficacy of the drugs against Covid-19, the Ministry of Health last week released a document with guidelines for the use of chloroquine.
The drug was the cause of discord between two former Ministers of Health and President Jair Bolsonaro. Luiz Henrique Mandetta and Nelson Teich, both doctors, alerted to the drugs’ side effects, but Bolsonaro argued for their use against Covid-19.
Mandetta was dismissed; Teich resigned less than a month after taking office. In addition to the chloroquine issue, the two ex-Ministers differed from the President regarding social isolation.
Soon after the Brazilian government released the document, which recommended the use of the drugs against Covid-19, Brazilian medical experts issued notes against the decision.
Both WHO and PAHO, the organization’s branch in the Americas, also reiterated that they recommend neither chloroquine nor hydroxychloroquine to treat Covid-19 outside clinical trials.
Secretary says WHO study is not sufficient for Brazil to review guidelines on chloroquine use for Covid-19 treatment
A spokesperson for the Ministry of Health said on Monday, May 25th, that the portfolio maintains the guidelines for the use of chloroquine and hydroxychloroquine in the treatment of Covid-19, even though the World Health Organization (WHO) has suspended trials with the drug after it was found to increase the risk of death.
“We are very calm and serene about the guidelines that provide doctors with the autonomy to offer this treatment to patients who wish to receive it,” said Mayra Pinheiro, secretary of Labor Management and Health Education.
According to Pinheiro, the portfolio follows 216 protocols for the use of chloroquine in the treatment of the disease in countries such as the United States, Turkey, and India. In addition, she said that the study that served as the basis for the WHO’s decision, published in “The Lancet”, does not have an “acceptable methodology to serve as a reference”.

