RIO DE JANEIRO, BRAZIL – The World Health Organization (WHO) is celebrating the recent approval by European drug regulators of the first vaccine against the Ebola virus.
It is “a victory for public health and a testament to the unprecedented cooperation between dozens of experts around the world,” according to the United Nations agency.
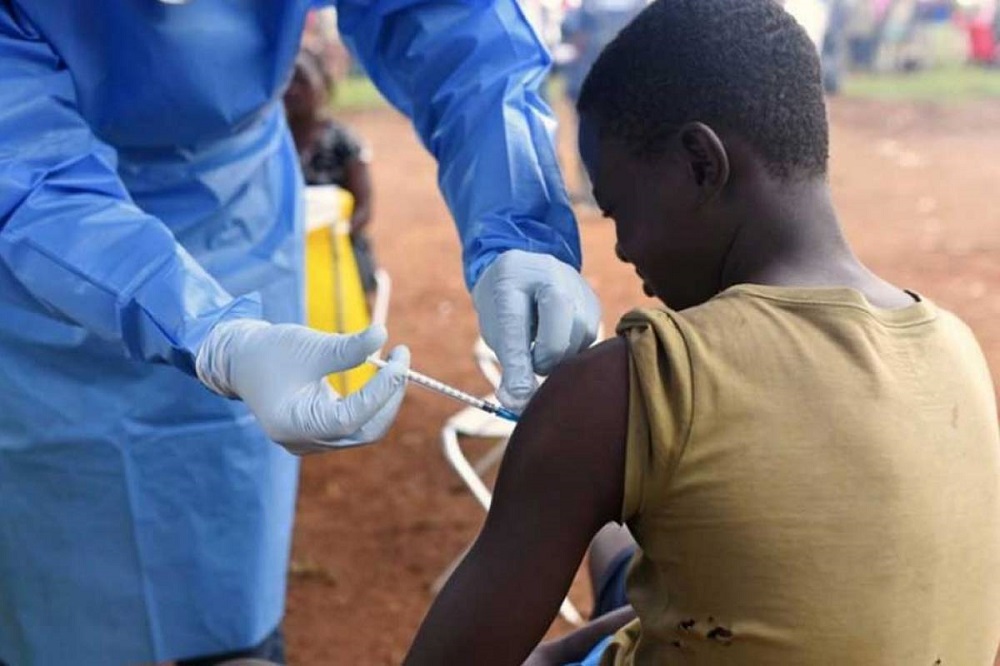
The European Medicines Agency (EMA) has released, for the first time, an Ebola vaccine tested in Guinea-Conakry (Africa), the country with the largest epidemic in history caused by the virus.
The product authorized by the EMA was initially developed with the brand by the American company Merck & Co.
More than 236,000 people have already been vaccinated. The number includes more than 60,000 frontline health professionals in the Congolese territory and in neighboring countries Uganda, South Sudan, Rwanda, and Burundi.
Regulations
The new vaccine is already being applied under the WHO’s emergency guidelines to protect people against the spread of the Ebola virus, which has killed over 2,100 people in Congolese territory since August last year.
According to WHO Director-General Tedros Ghebreyesus, the product has already “saved many lives in the current Ebola outbreak, and the decision by the European regulator will finally help to save many more”.
Support for research
The head of the UN agency said he was proud of the role WHO played in the vaccine development process, “from supporting research to testing” in Guinea-Conakry in 2015. The current Ebola outbreak is the second largest in history, after the epidemic that killed more than 11,300 people in West Africa between 2013 and 2016.
WHO has announced that it is working with the Global Alliance for Vaccines and the United Nations Children’s Fund (UNICEF) in this area, expecting that in coming years there will be greater demand for Ebola vaccines during and between outbreaks.
Global Safety Plan
The goal is to develop a Global Plan for Vaccine Safety because “greater supply capacity and several manufacturers will be needed in the short and medium-term to meet this demand and ensure the drug’s safety,” says the UN agency.
There are currently eight Ebola vaccines in the clinical evaluation process. WHO is working with partners to establish a coordinated government framework at the international level to ensure access to the product according to risk criteria.
WHO will also be expected to manage the stocks “because supply will remain limited until greater manufacturing capacity is created or other vaccines are licensed”.
Source: Agência Brasil

