RIO DE JANEIRO, BRAZIL – The clinical trial of CoronaVac, the vaccine developed by the Butantan Institute in partnership with biopharmaceutical company Sinovac Life Science, has reached its final stage.
According to information from the government of São Paulo and the Butantan Institute, the results will be released in the first week of December and the estimate is that 46 million doses will be available in Brazil by January 2021.
The results will be submitted by the independent International Committee in the first week of December for the National Health Regulatory Agency (ANVISA) to review the vaccine‘s verification report.
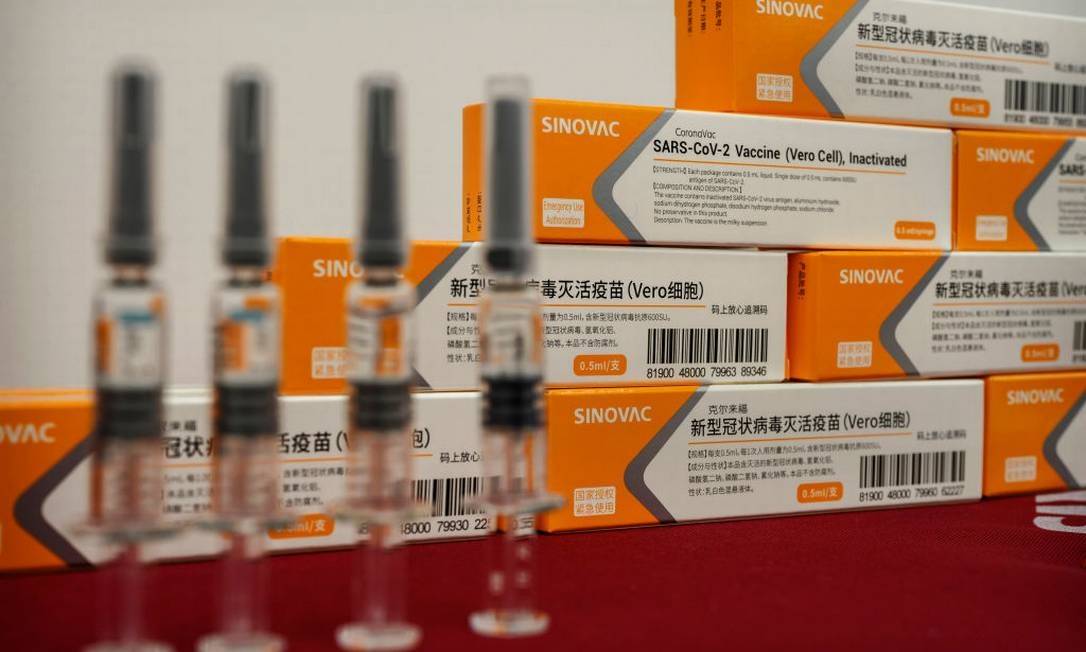
Trials in Brazil have been coordinated by Butantan since July, in 16 scientific research centers spread across seven Brazilian states and the Federal District, with 13,000 volunteers involved. Last week, the first batch with 120,000 doses reached São Paulo.
Last Tuesday, November 17th, the results of the preceding phase of the CoronaVac’s clinical trials were published by the prestigious Lancet scientific journal. The published report showed that the vaccine is safe and is able to produce an immune response in the body 28 days after its administration in 97% of cases.
On November 23rd, British pharmaceutical company AstraZeneca announced that the vaccine the laboratory has been developing against the novel coronavirus could be 90% effective, with no serious side effects.
Developed by Oxford University, it has reached this rate of prevention when administered at half a dose and, at least one month later, a full dose, according to data from an advanced stage clinical trials conducted in the United Kingdom and Brazil. The pharmaceutical company will have 200 million doses of the vaccine by the end of this year, with 700 million doses ready globally by the end of the first quarter of 2021.
American Pfizer announced last Wednesday, November 18th, that the final results of the advanced stage trials of its vaccine show that the immunizer is 95% effective, that it has all the safety data required for two months, and that it would request emergency use authorization in the United States within a few days.
According to Pfizer, the efficacy of the vaccine developed in partnership with the German BioNTech was consistent in age and ethnic demography data, and there were no major side effects.
Moderna, its competitor, released preliminary data on its vaccine on Monday, November 16th, showing similar efficacy.
Source: Agência Brasil

